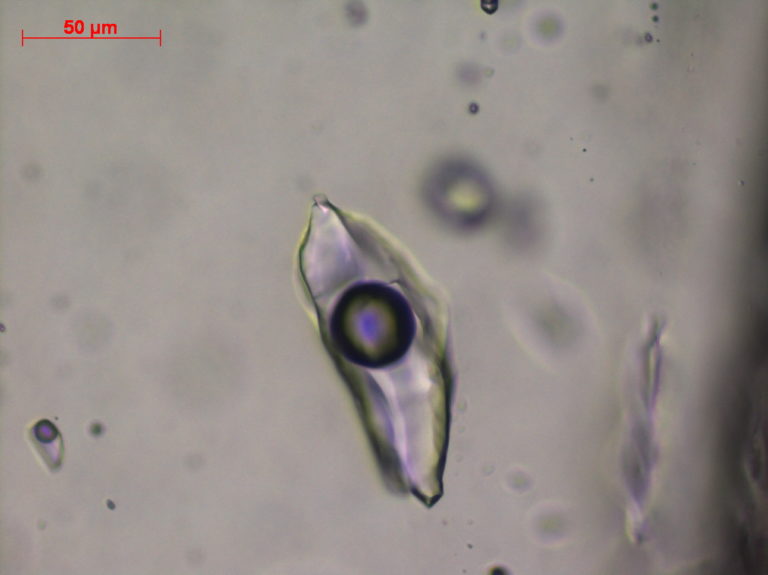
Aqueous liquid phase
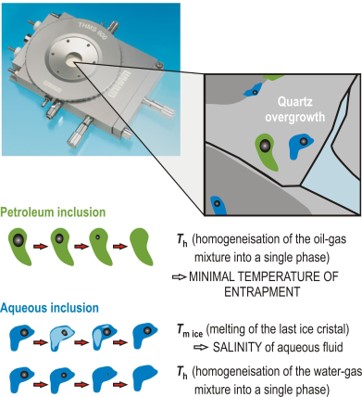
- The final ice melting temperatures (Tm ice) of aqueous fluids are translated into salinity.
- In the case of fragile minerals, Raman analysis of the water phase can help us to approximate salinity, taking into account mineral optical properties.
- The nature of solids, trapped in inclusions at ambient or low temperatures, can be determined.
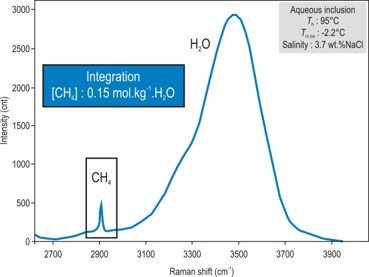
- Characterisation of dissolved gas (CH4, CO2, H2S, H2, O2, N2).
- Quantification of dissolved CH4, CO2 and H2S.
- Gas speciation can be evidenced (HCO3-, CO3=, HS-, SO4=, HSO4-,…)
Gas phase
- Phase transition inside gas inclusions gives information on gas nature and concentration
- Determination of the P-V-X properties of binary or ternary mixtures of CH4, CO2, and N2

Oil liquid phase
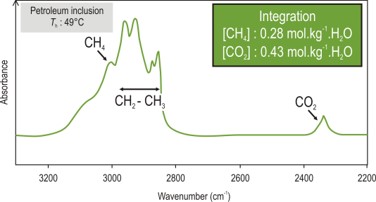
- Quantitative analysis (CH4, CH2, CH3, CO2) with FT-IR.
- Phase transition (L1+L2+V–>L+V) in hydrocarbons reveals the presence of gas condensates.
- Chemical mapping of inclusions can be realized at the scale of 1 micron.